Page 23 of 38.

Fda central document room.
Fda noted that this is the first time a contractor has handled the documents room.
Formal meetings with fda submit formalarchival submissions to the central document room submit desk paper copies of meeting packages to the regulatory project manager.
Abbreviated new drug applications andas.
Dhhsfdacderond office of drug evaluation iv 10903 new hampshire ave wo22 stop 5411 silver spring md 20903.
Rockville md based firm maxima will operate the fdas nda and ind receiving room effective march 31.
For the nda you should send the initial paper submission to the central document room.
Fda central document room attn.
For ind nda submissions with assigned.
You should send all other submissions to the appropriate division document room for g4707dftdoc.
2 for biological products regulated by cder.
20705 1266 please use the following attention lines with this address.
Maxima will be responsible for checking the completeness of applications accessing drug master files and forwarding the applications to appropriate agency.
Complaints should be sent to the fda central document room at the following address.
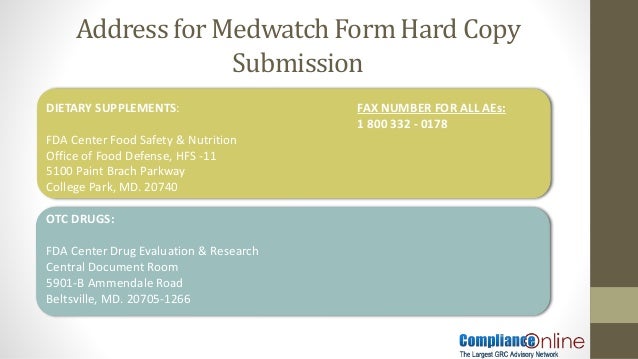
Fdacenter for drug evaluation and research cder central document room cdr 5901 b ammendale road beltsville md 20705 1266.
Click next to continue.
How to submit a meeting request to cder.
Submit formalarchival submissions to the central document room.
Patent information will be considered to be submitted to fda for purposes of paragraph d3 of this section as of the earlier of the date the information submitted on form fda 3542 is date stamped by the central document room or officially received by fda in an electronic format submission that complies with 31450l5.
Regulatory submissions central document room.
Food and drug administration center for drug evaluation and research office of prescription drug promotion 5901.
Engaging with the fda during new drug development.
Please use one of the attention lines from below 5901 b ammendale rd beltsville md.
The sponsor should contact the review division they plan to submit to and follow their advice.

Marktmeinungen Pictet Asset Management Altii Fondsportal

Clinical Investigator How Do I Put Together An Ind

Analysis Of Fda Enforcement Reports 2012 2019 To Determine

What Is A Drug Master File Ppt Download

Pdf Enhancing The Incorporation Of The Patient S Voice In

The Kis Foundation Inc Publications Facebook
Implant Files Medtronic Unter Schock

037 Status Update Loi 01142019

Administrative And Correspondence Documents

Ictr Ddrs Ind

Dokumentenlenkung Software Vergleiche Preise Top

Marktmeinungen Altii Fondsportal

Central Admixture Pharmacy Services Inc Valley View Oh

Participation Of Women In Clinical Trials Supporting Fda

Institution Logo

Guardian Pharmacy Services Dallas Tx 483 Issued 04 20 2018

Clinical Investigator How Do I Put Together An Ind

About Us Shandong Binzhou Zhiyuan Biological Technology Co

Ishwaria Subbiah Md Ms On Twitter Us Fda Cder S

Approval Letter

Various Radioeins Sessions Vol 4 Lp Pop Rock

Prozessleitsysteme Pls Yokogawa Deutschland Gmbh

Untitled

Kaolin Red Brown Soil Powder Pyrophyllite Powder

Clinical Investigator How To Put Together An Application

Expression Of Drug Transporters In Human Kidney Impact Of

Kongress Fur Infektionskrankheiten Und Tropenmedizin

Fda Gives Clovis Oncology S Rubraca A Priority Review Nasdaq

Innoveix Pharmaceuticals Inc Addison Tx 483 Issued 09 12 2019

Federal Register Abbreviated New Drug Applications And

Petition Tell The Fda To Approve The Drug Aducanumab For

Pdf Regulatory Requirements For Filing An Investigational

Inclusion Of Pregnant And Breastfeeding Women In Research

Guardian Pharmacy Services Dallas Tx 483 Issued 04 20 2018

Analysis Of Fda Enforcement Reports 2012 2019 To Determine

Structured Product Labelling By Pharma Student Issuu

Untitled

Formal Fda Meeting Request Guidance And Template Pdf Free

How To Submit A Meeting Request To Cder

Guardian Pharmacy Services Dallas Tx 483 Issued 04 20 2018

Structured Product Labelling By Pharma Student Issuu
Archiv

Prozessleitsysteme Pls Yokogawa Deutschland Gmbh

Usp Standard Pharmaceutical Grade Excipient Cas 182410 00 0 Betadex Sulfobutyl Ether Sodium Buy 182410 00 0 Sbecd Cyclodextrin Product On

Qualgen Llc Edmond Ok 483 Issued 09 13 2018

Dmf Drug Master File
Fda Adverse Event Reporting Requirements For Otc Drugs

Adrian S Weather Competitors Revenue And Employees Owler

Little Known Makers Of Generic Drugs Played Central Role In

Market Statements Erste Asset Management Gmbh Altii Fund
Informationen Fur Arzte Lost Voices Stiftung Hilfe Fur

Ppt A Tale Of Four Inds Powerpoint Presentation Free

Remote Health Solutions Telemedicine Remote Patient

Introduction To Dmf

The Impact Of Remote Monitoring On Cras Central Monitoring

Chungjin Biotech
Evosep Literature Room Your Shortcut To Our Knowledge Base

Is Your Software A Medical Device Raps

Archiv

Media Room Ccts

University Of Pennsylvania School Of Medicine

Document 15530481

Kaltumformung Aluminium Folie Fur Dichtung Alufolie Buy Kaltumformung Aluminiumfolie Alufolie Kaltumformung Aluminiumfolie Dichtung Alufolie Product

Guardian Pharmacy Services Dallas Tx 483 Issued 04 20 2018

Orthocure Home Facebook

Fda 21 Cfr Part 11 Compatible Hmi Scada Software Zenon

Translation Of Proteomic Biomarkers Into Fda Approved Cancer

Ptp Aluminum Foil Alu Alu Foil Tropical Blister Foils Al

美国fda Dmf备案 Crospovidone 产品资质重庆斯泰克瑞登梅尔材料

Vi Cell Blu Zellzahl Und Zellvitalitat Beckman Coulter

2017 Cardiovascular And Stroke Endpoint Definitions For

Ppt Overview Of Fda S Regulatory Framework For Pet Drugs
Fda S Framework For Regulating Regenerative Medicine Will

Step By Step Process For Ind Sponsor Sponsor Investigator

Analysis Of Fda Enforcement Reports 2012 2019 To Determine

Ranitidine Recall Issued Following Discovery Of Ndma

Cantrell Drug Company Little Rock Ar 483 Issued 08 22 2018

Ectd Module 1 From Submission To Reviewer Ppt Download

Drug Master Files Global Perspectives Iii Symposium

Gastroparesis How To Obtain Domperidone And Information
Pdf Dmf Filing In Us Europe And Canada

Helmholtz Open Science Workshop Elektronische Laborbucher
Energies Free Full Text Iso 50001 2018 And Its

Where To Send Ectd Sample Materials
.png)
Fda S Framework For Regulating Regenerative Medicine Will

Inside Story For Review Of Dmf And Dossiers By Regulatory

Federal Register Abbreviated New Drug Applications And

Reprocessing Of Single Use Devices Infection Control

Fda 2013 Clinical Investigator Training Course How Do I Put

Drug Master Files Ppt Video Online Download

The Electronic Regulatory Submission

Parasitic Infections In Solid Organ Transplantation

Guardian Pharmacy Services Dallas Tx 483 Issued 04 20 2018

Silicagel Products Mfg Co Manufacturer From Jodhpur

Meetings With Cder Judit Milstein Pdf Free Download

Tatigkeitsbericht Des Max Planck Instituts Fur Innovation

U S Public Health Service Pharmacist Professional Advisory

Structured Product Labelling By Pharma Student Issuu



































































.png)











